A Bar Domain-mediated Autoinhibitory Mechanism for Rhogaps of the Graf Family
Introduction
The molecular events that stimulate platelet adhesion, aggregation and thrombus formation are crucial to platelet function, both in haemostasis and thrombosis. It has been well documented that platelet regulation at a molecular level is a finely balanced system crosstalk between different signalling pathways, resulting in events such every bit phosphorylation, Catwo+ fluctuation, lipid modification and more (1). These processes are regulated and coordinated interdependently by small GTPases, allowing for the rapid alterations seen in the platelet cytoskeleton and overall platelet morphology upon activation (2–four). Approximately 8% of all proteins identified in platelets are pocket-sized GTPases and their regulators (5). Despite the Rho family of minor GTPases containing over 20 members, RhoA, Rac1 and Cdc42 are considered the primary drivers in the dynamic cytoskeletal reorganisation procedure, leading to the evolution of filopodia and lamellipodia (six). RhoA, Rac1 and Cdc42 accept likewise been linked with other processes in platelet activation such as platelet granule release (2), clot retraction (vii) and integrin activation via crosstalk with another pocket-size GTPase, Rap1 (8, ix). These proteins bicycle between their inactive Gross domestic product-bound and active GTP-bound states and this cycling of activation is facilitated by regulatory proteins known every bit GTPase-activating proteins (GAPs–inhibitory) and guanine nucleotide exchange factors (GEFs – activatory) (10–12). GAPs catalyse the extremely ho-hum intrinsic GTPase activity of Rho GTPases, terminating GTPase signalling (13), while GEFs facilitate the dissociation of GDP to GTP via their catalytic domains (10) (Figure 1).
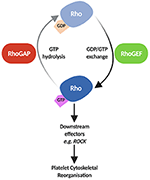
Effigy 1. Schematic of Rho GTPase regulation by RhoGAPs and RhoGEFs. In their inactive state, Rho GTPases such as RhoA, Rac1 and Cdc42 are Gross domestic product-bound. GEFs facilitate the dissociation of Gdp and bounden of GTP, activating Rho GTPases allowing them to interact with downstream effectors such as Rho Kinase (ROCK), Filamin-A, and Wiskott-Aldrich syndrome poly peptide (WASp) leading to reorganisation of the platelet cytoskeleton (two, 3). GAPs later on catalyse the hydrolysis of GTP to Gross domestic product, reverting the Rho GTPase to its inactive land, inhibiting Rho GTPase signalling. Paradigm created using BioRender.
While >70 RhoGAPs and >70 RhoGEFs (14, 15) have been identified in mammalian cells, platelets have been shown to express approximately 22 RhoGAPs and 27 RhoGEFs based on platelet proteomic analyses to date (Tabular array ane) (5, xviii, 20). However, few of these have been fully characterised in platelets, with detailed analysis on their role in platelet function severely lacking (14). Here, we provide an overview of the existing knowledge of identified RhoGAPs and RhoGEFs in platelets, including insights into their function, regulation and their role in platelet biology.
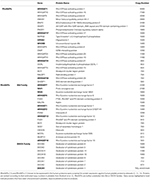
Table 1. Rho GTPase-activating proteins (RhoGAPs) and Rho guanine nucleotide commutation factors (RhoGEFs) expressed in human platelets.
RhoGAPs in Platelets
Unduly more (two-to-3 fold) GAPs are plant in the homo proteome compared to small GTPases and a number of theories have attempted to explain this difference (13). I theory is many GAPs exhibit specificity toward specific small GTPases. A caveat to this is many GAPs have been shown to exhibit some caste of regulatory power to various small-scale GTPases. A recent written report by Müller et al. (12) has highlighted how RhoGAPs are more than "promiscuous" than RhoGEFs regarding the number of GTPases which they tin can regulate. Another theory is the majority of GAPs are large, multi-domain molecules with functional roles in addition to their GAP activeness, meaning they act as conduits of betoken integration for various signalling pathways (13). Examples include the RhoGAP Bcr that possesses both GAP and Global environment facility domains targeted to Rac1 and Cdc42, respectively (21). Another case is ARHGAP17 which expresses a PDZ binding domain, known to regulate multiple biological processes and betoken transduction systems (22). Regulation of Rho GTPases by RhoGAPs in platelets remains poorly understood, with few of the approximately 22 RhoGAPs expressed in platelets having been thoroughly characterised. Platelets accept previously served as a model system for investigations into RhoGAPs, a prime instance being p50RhoGAP (ARHGAP1), a founding fellow member of the RhoGAP family unit. First identified in platelets, p50RhoGAP was shown to have loftier specificity for Cdc42 (23) and after RhoA and Rac1 in mammalian cell lines (21).
One of the first RhoA-specific GAP proteins characterised in platelets was p190RhoGAP (ARHGAP35), where its activity was stimulated during platelet activation via Src-family unit kinases (SFKs) inhibiting RhoA and thus facilitating platelet spreading (24). This is followed past inhibition of SFKs leading to an increase in RhoA-GTP levels promoting clot retraction (25). Activation of c-Src has also been suggested to mediate p190RhoGAP phosphorylation causing termination of RhoA signalling (24). Building on this, Flevaris et al. (25, 26) showed release of intracellular Caii+ activated calpain proteases which cleave the integrin β3 subunit (activating c-Src), resulting in p190RhoGAP inhibition and RhoA reactivation to promote jell retraction.
Oligophrenin-1 (OPHN1) is one of the few RhoGAPs to have been studied extensively in platelets (27). Aberrant RhoA activation coupled with defective adhesion and lamellipodia formation was shown in platelets from OPHN1−/− murine models (27). OPHN1 was also shown to localise to actin-rich regions in platelets where it exhibited regulatory roles in stress fibre, filopodia and lamellipodia formation, implicating information technology in the straight regulation of RhoA, Rac1 and Cdc42 (28). OPHN1 may take a part in platelet activation straight. OPHN1−/− murine platelets exhibit abnormal RhoA hyperactivation and significant increases in thrombus formation both ex- and in vivo (27). These findings propose aberrant regulation of Rho GTPases significantly impacts the ability of platelets to attach and undergo archetype shape change responses following stimulation of platelet activation (6).
ARHGAP17 (Nadrin) has been previously reported as a RhoGAP for Rac1 and Cdc42 in rat neuronal cells (29) and is described as a regulator of GAP activity through a machinery of auto-inhibition (28). ARHGAP17 was shown to relocalise, similarly to OPHN1, to actin-rich regions within platelets, suggesting a cytoskeletal regulatory role for ARHGAP17 (30). Furthermore, information technology was shown to exhibit preferences for specific Rho GTPases dependent on the ARHGAP17 isoform being assessed (xxx). Nagy et al. (31) found PKA/PKG mediated phosphorylation of S702, which lies in a proline rich and intrinsically disordered region of the protein, facilitated CIP4 binding. CIP4 is a Rac1 effector involved in lamellipodia and filopodia formation. CIP4/ARHGAP17 dissociation occurred upon PKA activation and coincided with decreases in Rac1-GTP levels, however, a straight correlation between Rac1 inhibition and CIP4 binding could not be confirmed using a S702A mutant (31).
Myo9b (MyoIXb) is a member of the mammalian class 9 myosins alongside Myo9a, which are unique amongst myosins due to the location of a GAP catalytic domain in the C-concluding tail region (32). Myo9b is found primarily in allowed cells (33, 34) and has been shown to exhibit loftier RhoA specificity. The Myo9b rat homologue myr5 inhibits Cdc42 and Rac1, but at levels 100-fold (Cdc42) or 1000-fold (Rac1) greater than required for RhoA inhibition (35). It was too shown that Myo9b deficient prostate cancer cells and murine macrophages express higher levels of phosphorylated MLC, indicative of increased RhoA-GTP levels (36, 37). We previously institute PKA/PKG phosphorylate Myo9b at S1354 in platelets enhancing Myo9b GAP activity leading to reduced RhoA-GTP levels. Myo9b phosphorylation, therefore, may contribute to local cyclic nucleotide-mediated control of RhoA and the actin/myosin cytoskeleton in platelets (38). Interestingly, in the same study we observed agonist-induced Myo9b phosphorylation suggestive of cAMP-independent PKA phosphorylation, previously proposed as an inhibitory feedback mechanism during thrombin- and collagen-induced platelet activation (38).
ARHGAP21 has previously been found to inhibit RhoA, RhoC (39) and Cdc42 in glioblastoma cell lines (xl). Further, it has likewise been shown to play roles in various cytoskeletal processes such as cell migration (39) and adhesion (40) and stress fibre germination (41). Haploinsufficient (ARHGAP21+/−) mice were originally characterised as having decreased platelet counts and increased platelet volumes (42). Recently ARHGAP21 has been investigated equally a key regulatory protein in megakaryocyte differentiation and platelet formation. ARHGAP21+/− murine platelets exhibit enhanced thrombin-induced platelet aggregation, highlighting an increased thrombin sensitivity (39). ARHGAP21+/− platelets also have increased P-selectin expression and increased levels of active RhoA and Cdc42 as well as enhanced thrombus formation (39).
The IQ-domain containing GAPs, IQGAP1 and IQGAP2, were reported to stabilise Rac1 and Cdc42 by binding to them straight [reviewed in (43)], yet these GAPs do not express a true RhoGAP domain or exert whatsoever GTPase role (6). Interestingly, Bahou et al. (44) showed thrombin, but not collagen handling induced the relocalisation of IQGAPs to the platelet cytoskeleton, specifically the cytoskeleton of filopodia. This suggests IQGAPs are activated downstream of the GPCRs PAR1/PAR4 merely not GPVI.
The above discussed RhoGAPs are currently the simply ones to accept been investigated in particular in platelets. Aside from these identified RhoGAPs, there are potentially many more expressed in platelets, as platelet proteomic and transcriptomic studies accept shown (5, 45).
RhoGEFs in Platelets
In dissimilarity to RhoGAPs, there are slightly more studies which characterise functions and roles of specific RhoGEFs in platelets. Humans limited approximately 81 RhoGEF proteins, exceeding the number of potential target small GTPases by a ratio of nearly iv:one (15). These RhoGEFs are classed into two distinct families: the Dbl (~lxx members) and Dock (~11 members) families. Diffuse B-cell lymphoma (Dbl) GEFs are characterised their classic tandem domain structure of a Dbl homology (DH) catalytic Gef domain, and the pleckstrin homology (PH) domain. The PH domain primarily stabilises the DH domain assuasive it to catalyse the Gross domestic product-to-GTP switch (46), amidst other functions such as phosphoinositide binding (47). Each member of the Dbl family contains other singled-out domains and motifs which facilitate their interaction with other proteins, cellular structures and make them candidates for various postal service-translational modifications (17). Members of the dedicator of cytokinesis (Dock) family are noted for the catalytic Dock homology region 2 (DHR2) GEF domain and the DHR1 domain, located C-terminally to DHR2 and involved in membrane localisation (48). Dbl family GEFs are known to showroom varied specificities to Rho GTPases, whereas the Dock family are thought to exist restricted to Rac/Cdc42 regulation [reviewed in (49–51)]. Although, recent work has shown reduced RhoA activity due to downregulation of Dock1 in triple negative breast cancer epithelial cells (52). Further, a weak interaction of Dock x with RhoF and RhoG in vitro has also been reported (53). Goggs et al. (3, 54) using a RhoG-GTPγS pull-downwardly proteomic arroyo found platelets limited Dock1, Dock5 and Dock10 although their role in platelet function, indeed the role of the Dock family of GEFs, remains poorly understood. RhoGEFs of the Dbl family are the focus of the nowadays review.
One of the all-time characterised RhoGEFs in platelets is the RhoA-specific ARHGEF1 (p115RhoGEF) (2). Downstream of GPCR stimulation, Kα13 was shown to bind ARHGEF1 direct through the One thousandα13 switch region 1 (SRI) thus promoting ARHGEF1 mediated RhoA activation (55). Furthermore, a recent study using ARHGEF1 knockout mice institute significantly prolonged thrombus occlusion and increased tail haemorrhage times. These mice also displayed significantly adulterate platelet aggregation, αIIbβthree activation and granule release in response to a diverseness of platelet agonists (56). Interestingly, ARHGEF1 was reported to act as a RhoGAP for Gα13 via its regulator of G-protein signalling (RGS) domain, equally well as functioning as a RhoGEF for RhoA. This potentially reveals a system of negative feedback on RhoA-GTP signalling; ARHGEF1 interim as an intermediary in Yardα13 regulation of RhoA (57).
Global environment facility-H1 (ARHGEF2) was initially characterised as a Dbl RhoGEF that integrated microtubule dynamics to jail cell contractility (58). Gao et al. (59) found decreases in RhoA-GTP levels during murine megakaryocyte endomitosis corresponded to downregulation of GEF-H1 at mRNA and poly peptide levels. Exogenous expression of GEF-H1 was also plant to induce low ploidy in developing megakaryocytes (59). Aslan et al. (threescore) subsequently reported GEF-H1 every bit a substrate for p21 activated kinases (PAKs) and associates with Rac1-GTP in response to thrombin. We previously reported PKA/PKG phosphorylation of Global environment facility-H1 at S886 increases xiv-3-3β interaction, inactivating Gef-H1 and decreasing RhoA-GTP (38). In the aforementioned written report we found nocodazole-induced disruption of platelet microtubules increases RhoA-GTP levels but does not affect S886 phosphorylation or RhoA inhibition. This is noteworthy every bit GEF-H1 is the only GEF reported to localise at cell microtubules (61), giving more than insight into platelet cytoskeletal dynamics.
ARHGEF6 (αPIX, Cool-two), a Rac specific RhoGEF, was first identified in platelets (excluding proteomic studies) in 2013. Aslan et al. (60) successfully used Rac1-GTPγS to isolate ARHGEF6 (and ARHGEF7) from thrombin-activated human being platelets. The SH3 domain of ARHGEF6 was previously shown to interact with PAK1-3 in vitro (62), well known downstream effectors of Rac and Cdc42 in human being platelets (63). Nagy et al. (31) discovered ARHGEF6 is constitutively associated with GIT1 (ArfGAP1) in platelets. GIT1 besides contains a GAP domain specific for the small GTPase Arf6 meaning GIT1 could contribute to the suppression of Arf6-GTP levels occurring during platelet activation (64). In the same study, the authors showed PKA/PKG-mediated phosphorylation of S684 promoted 14-3-iii bounden to ARHGEF6, reducing Rac1-GTP levels (31).
The RhoA-specific ARHGEF10 was shown by Matushita et al. (65) to be associated with increased risk of atherothrombotic stroke via a specific single-nucleotide polymorphism. Murine ARHGEF10−/− platelets exhibited reduced aggregation in response to various agonists and protected mice from thrombus formation (66). Lu et al. (66) reported ARHGEF10−/− platelets show decreased Rock phosphorylation, representative of reduced Gα13-mediated RhoA activation. This study highlights a fundamental office of ARHGEF10 in RhoA regulation and normal platelet role.
ARHGEF12 (LARG) is member of the Dbl family of RhoGEFs which has been well characterised in platelets. Williams et al. (67) found ARHGEF12−/− murine platelets were unaffected during thrombopoiesis simply exhibited a significant reduction in assemblage and dumbo granule secretion in response to U46619 (TXA2 synthetic analogue) and PAR receptor stimulation but not ADP. Platelet spreading and adhesion in response to fibrinogen or collagen-related peptide (CRP) were also unaffected (67). This suggests ARHGEF12 is stimulated downstream of Mα13 coupled receptors. All the same, the authors plant ARHGEF12 deletion only affected basal RhoA activity not agonist-induced activity. Therefore, ARHGEF12 may be responsible for RhoA activity in resting platelets but (67). In dissimilarity, Zou et al. (68) reported ARHGEF12 was necessary for RhoA activation in platelets. MLC phosphorylation past ROCK (downstream of RhoA-GTP) was abolished, albeit in a global ARHGEF12 knockout mouse model. Zou et al. (68) too noted differences in platelet agonist handling times and dosages equally potentially being responsible for the observed increase in ARHGEF12-mediated RhoA activation. Despite the differences between these studies, both confirmed ARHGEF12 functions downstream of Thouα13 and is necessary for normal platelet part, however, the impact on RhoA activation warrants further confirmatory investigations.
The Vav subgroup of Dbl RhoGEFs, comprised of Vav1-3 are well documented in platelets. This subgroup take been described every bit exhibiting GEF part toward Rho, Rac and Cdc42 but have a particular affinity for Rac proteins (2, fifteen). Vav activation in platelets was reported downstream of thrombin and collagen stimulation. Thrombin-stimulated PAR signalling and collagen interaction with α2β1 induced tyrosine kinase mediated phosphorylation of Vav within 15 seconds (69). Interestingly, ADP and U46619 did not induce whatsoever detectable tyrosine kinase-mediated phosphorylation of Vav, suggesting Vav phosphorylation and activation was platelet agonist specific (69). Pearse et al. (70) reported a minor role for Vav1 in platelet function, specifically during the later stages of thrombin- or CRP-induced platelet assemblage. Subsequently studies past Pearce et al. (seventy, 71) assessed the role of Vav3 in conjunction with Vav1 and showed both were required for platelet spreading through PLCγ regulation by αIIbβthree (72). Vav1/3 take been shown to form a complex with some other Rac-specific Gef, P-Rex1, regulating signalling events during thromboinflammation (73). P-Rex1−/− platelets take adulterate aggregation and secretion in response to collagen and GPCR agonists (74) but do not have impeded platelet spreading (75). RhoGEFs such as TIAM1 (76), Sos1 (77) and TRIO (iii) take all been proposed to be expressed in platelets, however, their functional relevance however remains unclear.
Conclusions
In recent years, information technology has become apparent that activatory and inhibitory platelet signalling pathways do not office independently of 1 another (1). There is considerable overlap and crosstalk between both systems which human action together to regulate platelet function. Interestingly, this crosstalk is very often focused on the same proteins i.e., proteins that can exist differentially regulated past both platelet activators and inhibitors (1). Recent piece of work has shown how RhoGAPs and RhoGEFs function collectively with systems-level behaviour to manage Rho activity in specific cellular regions. Müller et al. (12) established a regulator-centric model of Rho regulation whereby the regulators (RhoGAPs and RhoGEFs) supply spatiotemporal information based on their location on specific cellular structures. This suggests RhoGAPs and RhoGEFs are primed to respond to localised regulation, allowing for expedient modification to diverse stimuli. We see evidence for this in RhoA regulation. Graessl et al. (78) reported Myo9b and Global environment facility-H1 form a network with RhoA and actin filaments, generating dynamic patterns of subcellular contractility in adherent U2OS cells.
The functional diverseness of Rho GTPases downstream of platelet signalling pathways highlights the need for regulators of these molecular mechanisms. RhoGAPs and RhoGEFs are centrally positioned as conduits for diverse interweaving activatory and inhibitory signalling pathways in platelets, contributing to effective haemostasis (1, 79). Rho GTPases also play roles in various pathologies (14) and targeting Rho GTPase pathways has been a focus of pharmacological advancements, particularly in potential cancer therapies (eighty), a contempo success being new direct RasG12 inhibitors against cancer (81). However, very few therapies that target Rho GTPase signalling accept been developed across clinical pretrials [reviewed in (82)].
Much effort has been spent on developing compounds which inhibit GDP/GTP commutation which suggests strategic targeting of RhoGAPs and RhoGEFs could lead to greater selectivity in targeted treatments. Examples of this include development of ARHGEF12 inhibitors (83) and the Rac1/Vav2 interaction inhibitor EHop-016 (84). Enquiry targeting RhoGAPs is limited, but the goal is to raise GTPase action. There is limited evidence for targeting the Chimaerin C1 domain of the Rac-specific Chimaerin RhoGAP family unit (85). C1-bounden pocket-size molecules may heighten chimaerin GAP activity (86) although potential for off-target effects is considerable as many proteins express C1 domains.
Presently, we are only beginning to sympathize the multifaceted roles RhoGAPs and RhoGEFs play in Rho GTPase regulation in platelets. Platelet proteomic studies (5, eighteen, 20) have provided a clearer picture of how many RhoGAPs and RhoGEFs are expressed in platelets, just characterisation studies focused on their specific function in platelets will be the key to providing new insights. Hereafter work should investigate pharmacological inhibition/activation of specific RhoGAPs and RhoGEFs in platelets, with the aim of identifying targets which tin function as anti-platelet therapies while preserving haemostatic function.
Author Contributions
SC conceptualised, researched, and wrote the article.
Conflict of Interest
SC is the Sanofi S.A. Newman Fellow in Haematology. Sanofi South.A. had no input into the preparation of the manuscript or determination to publish.
Publisher's Notation
All claims expressed in this commodity are solely those of the authors and practice not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may exist made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author wishes to thank Dr Albert Smolenski Physician for his valuable comments and help with the preparation of the manuscript and for introducing the author to RhoGAPs and RhoGEFs in the offset identify.
References
1. Nagy Z, Smolenski A. Cyclic nucleotide-dependent inhibitory signaling interweaves with activating pathways to determine platelet responses. Res Pract Thromb Haemost. (2018) two:558–71. doi: ten.1002/rth2.12122
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Burkhart JM, Vaudel M, Gambaryan S, Radau Due south, Walter U, Martens 50, et al. The first comprehensive and quantitative assay of homo platelet poly peptide limerick allows the comparative analysis of structural and functional pathways. Blood. (2012) 120:e73–82. doi: 10.1182/blood-2012-04-416594
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. (2012) 119:1054–63. doi: 10.1182/blood-2011-08-372193
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Stefanini L, Boulaftali Y, Ouellette TD, Holinstat Grand, Désiré L, Leblond B, et al. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler Thromb Vasc Biol. (2012) 32:434–41. doi: 10.1161/ATVBAHA.111.239194
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Müller PM, Rademacher J, Bagshaw RD, Wortmann C, Barth C, van Unen J, et al. Systems assay of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol. (2020) 22:498–511. doi: 10.1038/s41556-020-0488-ten
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Csepanyi-Komi R, Safar D, Grosz V, Tarjan ZL, Ligeti E. In silico tissue-distribution of homo Rho family GTPase activating proteins. Small-scale GTPases. (2013) 4:90–101. doi: 10.4161/sgtp.23708
PubMed Abstract | CrossRef Total Text | Google Scholar
17. Melt DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. (2014) 33:4021–35. doi: 10.1038/onc.2013.362
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Makhoul S, Walter Eastward, Pagel O, Walter U, Sickmann A, Gambaryan S, et al. Furnishings of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide. (2018) 76:71–80. doi: ten.1016/j.niox.2018.03.008
PubMed Abstract | CrossRef Full Text | Google Scholar
xix. Beck F, Geiger J, Gambaryan Due south, Veit J, Vaudel M, Nollau P, et al. Fourth dimension-resolved characterization of army camp/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. (2014) 123:e1–10. doi: 10.1182/blood-2013-07-512384
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Beck F, Geiger J, Gambaryan S, Solari FA, Dell'Aica 1000, Loroch S, et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Claret. (2017) 129:e1–e12. doi: 10.1182/blood-2016-05-714048
PubMed Abstract | CrossRef Total Text | Google Scholar
21. Ridley AJ, Self AJ, Kasmi F, Paterson HF, Hall A, Marshall CJ, et al. rho family GTPase activating proteins p190, bcr and rhoGAP testify distinct specificities in vitro and in vivo. EMBO J. (1993) 12:5151–60. doi: ten.1002/j.1460-2075.1993.tb06210.ten
PubMed Abstruse | CrossRef Full Text | Google Scholar
23. Hart MJ, Shinjo Chiliad, Hall A, Evans T, Cerione RA. Identification of the human platelet GTPase activating protein for the CDC42Hs protein. J Biol Chem. (1991) 266:20840–eight. doi: 10.1016/S0021-9258(18)54786-4
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Flevaris P, Stojanovic A, Gong H, Chishti A, Welch Due east, Du Ten, et al. molecular switch that controls prison cell spreading and retraction. J Cell Biol. (2007) 179:553–65. doi: 10.1083/jcb.200703185
PubMed Abstruse | CrossRef Total Text | Google Scholar
26. Flevaris P, Li Z, Zhang Grand, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated poly peptide kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. (2009) 113:893–901. doi: 10.1182/blood-2008-05-155978
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Fotinos A, Klier Yard, Gowert NS, Münzer P, Klatt C, Beck Southward, et al. Loss of oligophrenin1 leads to uncontrolled Rho activation and increased thrombus formation in mice. J Thromb Haemost. (2015) 13:619–30. doi: 10.1111/jth.12834
PubMed Abstruse | CrossRef Full Text | Google Scholar
28. Elvers M, Beck S, Fotinos A, Ziegler M, Gawaz Grand. The GRAF family member oligophrenin1 is a RhoGAP with BAR domain and regulates Rho GTPases in platelets. Cardiovasc Res. (2012) 94:526–36. doi: 10.1093/cvr/cvs079
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Furuta B, Harada A, Kobayashi Y, Takeuchi K, Kobayashi T, Umeda Grand. Identification and functional characterization of nadrin variants, a novel family of GTPase activating protein for rho GTPases. J Neurochem. (2002) 82:1018–28. doi: 10.1046/j.1471-4159.2002.01021.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
thirty. Brook S, Fotinos A, Lang F, Gawaz Chiliad, Elvers Thousand. Isoform-specific roles of the GTPase activating protein Nadrin in cytoskeletal reorganization of platelets. Jail cell Indicate. (2013) 25:236–46. doi: 10.1016/j.cellsig.2012.09.005
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Nagy Z, Wynne One thousand, von Kriegsheim A, Gambaryan S, Smolenski A. Cyclic nucleotide-dependent protein kinases target ARHGAP17 and ARHGEF6 complexes in platelets. J Biol Chem. (2015) 290:29974–83. doi: x.1074/jbc.M115.678003
PubMed Abstruse | CrossRef Full Text | Google Scholar
32. Bahler M. Form Ix myosins. In: Coluccio LM, editor. Myosins: A Superfamily of Molecular Motors. Dordrecht: Springer Netherlands (2008). p. 391–401. doi: 10.1007/978-1-4020-6519-4_13
CrossRef Full Text | Google Scholar
33. Wirth JA, Jensen KA, Mail service PL, Bement WM, Mooseker MS. Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J Cell Sci. (1996) 109:653. doi: 10.1242/jcs.109.3.653
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Müller RT, Honnert U, Reinhard J, Bähler M. The rat myosin myr 5 is a GTPase-activating protein for Rho in vivo: essential function of arginine 1695. Mol Biol Cell. (1997) 8:2039–53. doi: 10.1091/mbc.eight.ten.2039
PubMed Abstruse | CrossRef Full Text | Google Scholar
36. Makowska KA, Hughes RE, White KJ, Wells CM, Peckham M. Specific myosins control actin arrangement, cell morphology, and migration in prostate cancer cells. Cell Rep. (2015) 13:2118–25. doi: 10.1016/j.celrep.2015.11.012
PubMed Abstruse | CrossRef Total Text | Google Scholar
37. Hanley PJ, Xu Y, Kronlage M, Grobe K, Schön P, Song J, et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motion. Proc Nat Acad Sci. (2010) 107:12145–l. doi: 10.1073/pnas.0911986107
PubMed Abstract | CrossRef Total Text | Google Scholar
38. Comer S, Nagy Z, Bolado A, von Kriegsheim A, Gambaryan S, Walter U, et al. The RhoA regulators Myo9b and GEF-H1 are targets of cyclic nucleotide-dependent kinases in platelets. J Thromb Haemost. (2020) eighteen:3002–12. doi: 10.1111/jth.15028
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Lazarini 1000, Traina F, Machado-Neto JA, Barcellos KS, Moreira YB, Brandão MM, et al. ARHGAP21 is a RhoGAP for RhoA and RhoC with a role in proliferation and migration of prostate adenocarcinoma cells. Biochim Biophys Acta. (2013) 1832:365–74. doi: 10.1016/j.bbadis.2012.11.010
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Bigarella CL, Borges L, Costa FF, Saad STO. ARHGAP21 modulates FAK activity and impairs glioblastoma cell migration. Biochim Biophys Acta. (2009) 1793:806–sixteen. doi: 10.1016/j.bbamcr.2009.02.010
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Anthony D, Sin Y, Vadrevu Due south, Advant N, Day J, Byrne A, et al. ARHGAP21, promoting activation of RhoA post-obit angiotensin Two Type 1A receptor stimulation. Mol Cell Biol. (2011) 31:1066–75. doi: 10.1128/MCB.00883-10
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Xavier-Ferrucio J, Ricon L, Vieira G, Longhini AL, Lazarini K, Bigarella CL, et al. Hematopoietic defects in response to reduced Arhgap21. Stalk Prison cell Res. (2018) 26:17–27. doi: ten.1016/j.scr.2017.11.014
PubMed Abstract | CrossRef Total Text | Google Scholar
44. Bahou WF, Scudder L, Rubenstein D, Jesty J. A shear-restricted pathway of platelet procoagulant activity is regulated by IQGAP1. J Biol Chem. (2004) 279:22571–seven. doi: 10.1074/jbc.M402561200
PubMed Abstruse | CrossRef Full Text | Google Scholar
45. Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of man and mouse platelet transcriptomes. Blood. (2011) 118:e101–e11. doi: 10.1182/blood-2011-03-339705
PubMed Abstract | CrossRef Full Text | Google Scholar
fifty. Kukimoto-Niino One thousand, Ihara Grand, Murayama K, Shirouzu G. Structural insights into the minor GTPase specificity of the DOCK guanine nucleotide commutation factors. Curr Opin Struct Biol. (2021) 71:249–58. doi: ten.1016/j.sbi.2021.08.001
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Thompson AP, Bitsina C, Gray JL, von Delft F, Brennan PE. RHO to the DOCK for GDP disembarking: structural insights into the DOCK GTPase nucleotide exchange factors. J Biol Chem. (2021) 296:100521. doi: x.1016/j.jbc.2021.100521
PubMed Abstract | CrossRef Total Text | Google Scholar
52. Liang Y, Wang S, Zhang Y. Downregulation of Dock1 and Elmo1 suppresses the migration and invasion of triple-negative breast cancer epithelial cells through the RhoA/Rac1 pathway. Oncol Lett. (2018) 16:3481–8. doi: 10.3892/ol.2018.9077
PubMed Abstruse | CrossRef Total Text | Google Scholar
53. Ruiz-Lafuente N, Alcaraz-García Thousand-J, García-Serna A-M, Sebastián-Ruiz S, Moya-Quiles 1000-R, García-Alonso A-1000, et al. Dock10, a Cdc42 and Rac1 GEF, induces loss of elongation, filopodia, and ruffles in cervical cancer epithelial HeLa cells. Biol Open. (2015) iv:627–35. doi: ten.1242/bio.20149050
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Goggs R, Harper MT, Pope RJ, Savage JS, Williams CM, Mundell SJ, et al. RhoG protein regulates platelet granule secretion and thrombus germination in mice. J Biol Chem. (2013) 288:34217–29. doi: ten.1074/jbc.M113.504100
PubMed Abstract | CrossRef Total Text | Google Scholar
55. Huang J-Southward, Dong L, Kozasa T, Le Breton GC. Signaling through Gα13 Switch region I Is essential for protease-activated receptor 1-mediated homo platelet shape change, aggregation, and secretion. J Biol Chem. (2007) 282:10210–22. doi: 10.1074/jbc.M605678200
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Qasim H, Karim ZA, Hernandez KR, Lozano D, Khasawneh FT, Alshbool FZ. Arhgef1 plays a vital role in platelet function and thrombogenesis. J Am Heart Assoc. (2019) eight:e011712. doi: x.1161/JAHA.118.011712
PubMed Abstruse | CrossRef Full Text | Google Scholar
57. Kozasa T, Jiang 10, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase Activating Poly peptide for Thouα12 and Gα13. Science. (1998) 280:2109–eleven. doi: 10.1126/science.280.5372.2109
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange gene Global environment facility-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. (2002) 4:294–301. doi: 10.1038/ncb773
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Gao Y, Smith Eastward, Ker Eastward, Campbell P, Cheng E-c, Zou Due south, et al. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Prison cell. (2012) 22:573–84. doi: 10.1016/j.devcel.2011.12.019
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, et al. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Jail cell Physiol. (2013) 305:C519–C28. doi: 10.1152/ajpcell.00418.2012
PubMed Abstract | CrossRef Total Text | Google Scholar
61. Azoitei ML, Noh J, Marston DJ, Roudot P, Marshall CB, Daugird TA, et al. Spatiotemporal dynamics of Gef-H1 activation controlled by microtubule-and Src-mediated pathways. J Cell Biol. (2019) 218:3077–97. doi: 10.1083/jcb.201812073
PubMed Abstract | CrossRef Total Text | Google Scholar
62. Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, et al. PAK kinases are directly coupled to the PIX family unit of nucleotide commutation factors. Mol Cell. (1998) 1:183–92. doi: ten.1016/S1097-2765(00)80019-2
PubMed Abstract | CrossRef Total Text | Google Scholar
63. Crespin K, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/ii during the shedding of platelet microvesicles. Blood Coagul Fibrinolysis. (2009) 20:63–seventy. doi: 10.1097/MBC.0b013e32831bc310
PubMed Abstract | CrossRef Full Text | Google Scholar
64. van den Bosch MT, Poole AW, Hers I. Cytohesin-ii phosphorylation by poly peptide kinase C relieves the constitutive suppression of platelet dense granule secretion by ADP-ribosylation factor 6. J Thromb Haemost. (2014) 12:726–35. doi: 10.1111/jth.12542
PubMed Abstract | CrossRef Full Text | Google Scholar
65. Matsushita T, Ashikawa Thou, Yonemoto G, Hirakawa Y, Hata J, Amitani H, et al. Functional SNP of ARHGEF10 confers take a chance of atherothrombotic stroke. Hum Mol Genet. (2009) 19:1137–46. doi: 10.1093/hmg/ddp582
PubMed Abstruse | CrossRef Full Text | Google Scholar
66. Lu DH, Hsu CC, Huang SW, Tu HJ, Huang TF, Liou HC, et al. ARHGEF10 knockout inhibits platelet aggregation and protects mice from thrombus formation. J Thromb Haemost. (2017) fifteen:2053–64. doi: 10.1111/jth.13799
PubMed Abstract | CrossRef Total Text | Google Scholar
67. Williams CM, Harper MT, Goggs R, Walsh TG, Offermanns South, Poole AW. Leukemia-associated Rho guanine-nucleotide substitution factor is not critical for RhoA regulation, nonetheless is of import for platelet activation and thrombosis in mice. J Thromb Haemost. (2015) 13:2102–7. doi: 10.1111/jth.13129
PubMed Abstract | CrossRef Total Text | Google Scholar
68. Zou S, Teixeira AM, Yin M, Xiang Y, Xavier-Ferruccio J, Zhang P-10, et al. Leukaemia-associated Rho guanine nucleotide commutation factor (LARG) plays an agonist specific role in platelet function through RhoA activation. Thromb Haemost. (2016) 116:506–16. doi: 10.1160/TH15-11-0848
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Cichowski Thou, Brugge JS, Brass LF. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J Biol Chem. (1996) 271:7544–l. doi: 10.1074/jbc.271.13.7544
PubMed Abstract | CrossRef Full Text | Google Scholar
70. Pearce Air conditioning, Wilde JI, Doody GM, Best D, Inoue O, Vigorito Due east, et al. Vav1, just not Vav2, contributes to platelet assemblage by CRP and thrombin, only neither is required for regulation of phospholipase C. Blood. (2002) 100:3561–9. doi: 10.1182/claret.V100.ten.3561
PubMed Abstruse | CrossRef Total Text | Google Scholar
71. Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and vav3 take critical but redundant roles in mediating platelet activation by collagen. J Biol Chem. (2004) 279:53955–62. doi: x.1074/jbc.M410355200
PubMed Abstract | CrossRef Full Text | Google Scholar
72. Pearce Air-conditioning, McCarty OJ, Calaminus SD, Vigorito E, Turner M, Watson SP. Vav family proteins are required for optimal regulation of PLCgamma2 by integrin alphaIIbbeta3. Biochem J. (2007) 401:753–61. doi: 10.1042/BJ20061508
PubMed Abstract | CrossRef Full Text | Google Scholar
73. Pan D, Amison RT, Riffo-Vasquez Y, Spina D, Cleary SJ, Wakelam MJ, et al. P-Male monarch and Vav Rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood. (2015) 125:1146–58. doi: 10.1182/blood-2014-07-591040
PubMed Abstruse | CrossRef Full Text | Google Scholar
74. Aslan JE, Spencer AM, Loren CP, Pang J, Welch HC, Greenberg DL, et al. Characterization of the Rac guanine nucleotide exchange cistron P-Rex1 in platelets. J Mol Bespeak. (2011) 6:11. doi: 10.1186/1750-2187-vi-eleven
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Qian F, Le Breton GC, Chen J, Deng J, Christman JW, Wu D, et al. Role for the guanine nucleotide exchange gene phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler Thromb Vasc Biol. (2012) 32:768–77. doi: ten.1161/ATVBAHA.111.243675
PubMed Abstract | CrossRef Full Text | Google Scholar
76. O'Toole TE, Bialkowska K, Li X, Fob JEB. Tiam1 is recruited to β1-integrin complexes by xiv-3-3ζ where it mediates integrin-induced Rac1 activation and motility. J Cell Physiol. (2011) 226:2965–78. doi: 10.1002/jcp.22644
PubMed Abstract | CrossRef Full Text | Google Scholar
77. Robinson A, Gibbins J, Rodriguez-Linares B, Finan P, Wilson L, Kellie Due south, et al. Characterization of Grb2-binding proteins in homo platelets activated by Fc gamma RIIA cross-linking. Blood. (1996) 88:522–30. doi: x.1182/blood.V88.ii.522.bloodjournal882522
PubMed Abstract | CrossRef Total Text | Google Scholar
78. Graessl M, Koch J, Calderon A, Kamps D, Banerjee S, Mazel T, et al. An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J Cell Biol. (2017) 216:4271–85. doi: 10.1083/jcb.201706052
PubMed Abstract | CrossRef Total Text | Google Scholar
79. Yu H, Kim PM, Sprecher Due east, Trifonov V, Gerstein M. The importance of bottlenecks in protein networks: correlation with gene essentiality and expression dynamics. PLoS Comput Biol. (2007) 3:e59. doi: 10.1371/journal.pcbi.0030059
PubMed Abstract | CrossRef Total Text | Google Scholar
83. Shang 10, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen K, Duhr Southward, et al. Rational pattern of modest molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. (2012) 19:699–710. doi: 10.1016/j.chembiol.2012.05.009
PubMed Abstruse | CrossRef Full Text | Google Scholar
84. Montalvo-Ortiz BL, Castillo-Pichardo L, Hernández E, Humphries-Bickley T, De La Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. (2012) 287:13228–38. doi: 10.1074/jbc.M111.334524
PubMed Abstract | CrossRef Full Text | Google Scholar
85. Caloca MJ, Garcia-Bermejo ML, Blumberg PM, Lewin NE, Kremmer Eastward, Mischak H, et al. beta2-chimaerin is a novel target for diacylglycerol: binding properties and changes in subcellular localization mediated by ligand binding to its C1 domain. Proc Natl Acad Sci U Due south A. (1999) 96:11854–9. doi: x.1073/pnas.96.21.11854
PubMed Abstract | CrossRef Total Text | Google Scholar
86. Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta. (2006) 1761:827–37. doi: 10.1016/j.bbalip.2006.05.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Glossary
A, alanine; ADP, adenosine diphosphate; Arf, ADP-ribosylation factor; ARHGAP, Rho GTPase-activating protein ARHGEF, Rho guanine nucleotide exchange factor; Bcr, breakpoint cluster region poly peptide; Catwo+, calcium ion; CalDAG-GEFI, Calcium and DAG-regulated guanine nucleotide commutation factor I; campsite, cyclic adenosine monophosphate; Cdc42, prison cell division control protein 42; CIP4, CDC42-interacting poly peptide iv; CRP, collagen-related peptide; C3G, RapGEF1; Dbl, diffuse B-cell lymphoma; DH, Dbl homology; DHR, dock homology region; DOCK, dedicator of cytokinesis; GAP, GTPase activating poly peptide; Gross domestic product, guanine diphosphate; GEF, guanine nucleotide exchange factor; GIT1, ArfGAP1; GP, glycoprotein; GPCR, 1000-poly peptide coupled receptor; GTP, guanine triphosphate; IQGAP, IQ calmodulin-bounden motif containing GTPase activating protein; LARG, leukaemia-associated Rho GEF (ARHGEF12); MLC, myosin light concatenation; Myo9b, unconventional myosin IXb; OPHN1, Oligophrenin-1; PAK, p21-activating kinase; PAR, protease activated receptor; PDE3A, phosphodiesterase 3A; PDZ, postal service synaptic density protein (PSD95), Drosophila disc large neoplasm suppressor (Dlg1), and zonula occludens-1 poly peptide (zo-1); PH, pleckstrin homology; PKA, protein kinase A; PKG, protein kinase G; PLCγ, phospholipase C gamma; P-Rex1, PI(3,4,5)P3-dependent Rac exchanger 1 protein; Rac1, Ras-related C3 botulinum toxin substrate 1; Rap1, Ras-related protein 1; RASA3, Ras GTPase-activating protein 3; RGS, regulator of k-poly peptide signalling; RhoA, Ras homology family member A; Rho GTPase, Ras homology family GTPase; Rock, Rho kinase; S, serine; SFK, Src family kinase; SH3, Src homology 3 domain; Sos1, son of sevenless homologue 1; Src, Proto-oncogene tyrosine-protein kinase Src; SRI, switch region1; TIAM1, T-lymphoma invasion and metastasis-inducing protein 1; TRIO, triple functional domain containing protein; TXA2, thromboxane A2; Vav, vav guanine nucleotide commutation gene; WASP, Wiskott–Aldrich syndrome protein.
Source: https://www.frontiersin.org/articles/10.3389/fcvm.2021.820945/full
0 Response to "A Bar Domain-mediated Autoinhibitory Mechanism for Rhogaps of the Graf Family"
إرسال تعليق